The womb is home to the most complex feat of human biology: the transformation from embryo to fetus to baby. But that magnificent conversion would be impossible without the placenta, the life-giving organ that the developing fetus is tied to via the umbilical cord. Even before a woman knows she’s pregnant, the placenta swells in size, poised to serve as the fetus’s kidneys and liver until the fetus has its own. The placenta starts to “breathe” for the fetus around 12 weeks in. Over a convoluted surface that grows large enough to cover a horse, fetal blood on one side soaks up oxygen from mom’s blood on the other. Her oxygen flows seamlessly into the fetus’s beating heart, brain, and limbs, and carbon dioxide from the fetus returns to the mother’s blood, to be exhaled in her breath.
Re-creating everything that happens inside the womb belongs firmly in the realm of science fiction. There’s still too much that scientists don’t know about the early stages of development, when fetal cells grow into organs, limbs, and tissues. But George Mychaliska thinks that creating an artificial version of the placenta, or at least replicating its most important function, is in reach. As a fetal and pediatric surgeon at the University of Michigan’s C.S. Mott Children’s Hospital, in Ann Arbor, he often sees premature babies who have left the womb too soon. Although modern medicine can save many of them, the chances of survival for extremely small preemies—those younger than 28 weeks, barely in their third trimester—remain slim. Of the survivors, many are left with long-term health problems. Lungs simply aren’t designed to breathe until the baby is close to full term, which is currently defined as 39 weeks, and even the gentlest techniques to assist breathing can damage the tissue.
“We’re in a catch-22 as baby doctors,” Mychaliska says. “If we don’t do anything, they die. If we want to save them… they may survive, but they likely will have varying degrees of lung disease from the treatment itself.”
For more than a decade, Mychaliska has been working on a solution: an artificial placenta to keep extremely young preemies alive until they can breathe on their own. Already, he’s proven that it can sustain premature lambs for several weeks. Building a breathing apparatus for a premature infant is not trivial, as the baby’s tiny size and fragile physiology pose both medical and engineering challenges. Mychaliska’s team has been adapting existing technology to work reliably with the skinniest of blood vessels and developing materials compatible with the unique biology of fetuses. Now, after several recent breakthroughs, Mychaliska thinks his team’s artificial placenta is only five years away from human trials.
His system is one of several designs under development around the world that aim to breathe for extremely premature babies. Some mimic the fetal environment by submerging the fetus in a fluid bath, which gets closer to the conditions of an artificial womb. Other designs rely on novel technology that attempts to mimic the way the lungs breathe.
As these devices move closer to clinical trials in humans, they’re also raising a number of ethical questions about where the technology—and where our society—is headed. Some bioethicists worry that opponents of abortion will seize on the speculative idea that artificial placentas could keep younger and younger preemies alive.
But researchers focus on the life-saving utility of this line of study. “It holds out a lot of hope for babies that are going to be born preterm,” says David Weinberg, who heads the Human Placenta Project at the National Institute of Child Health and Human Development, in Bethesda, Md. While cautioning that more testing is needed before these technologies can be used in the clinic, he’s impressed with the researchers’ ability to think through all the processes needed to keep the fetus viable: “It’s incredible.”
The idea of an artificial placenta goes back more than half a century. In 1953, U.S. surgeons operating on an adult patient’s heart successfully employed a heart-lung machine to externally oxygenate the patient’s blood. In the following decade, scientists were eager to try the new technology on preemies; after all, oxygenating blood outside the body is what the placenta does for the fetus. At the time, many hospitals didn’t even have intensive care units for sick newborns.
But in tests on premature lambs, those early oxygenators couldn’t sustain the animals for long, for reasons that aren’t entirely clear. Meanwhile, special ventilation techniques for newborns and drugs to boost their lung function began to emerge, helping thousands of premature babies survive. Interest in the artificial placenta waned.
By the turn of the millennium, a new type of oxygenator was being used in hospitals, in large part thanks to Robert Bartlett, a now-retired surgeon at the University of Michigan who’s known as the father of extracorporeal membrane oxygenation (ECMO). The earliest oxygenators worked by exposing blood directly to air, which could cause bubbles that blocked blood vessels and couldn’t support patients for long periods of time. ECMO, which relied on a new type of oxygenator, proved to be a longer-lasting and safer approach. Inside the oxygenator at the heart of the ECMO system, oxygen is pushed through bundles of hollow fibers made of plastic membrane, and it diffuses into the patient’s blood as the blood flows around the fibers. Red blood cells take up the oxygen, passing carbon dioxide back into the fibers. This technique has been used on tens of thousands of patients with lung or heart failure and has helped improved the chances for preemies.
Breath of Life
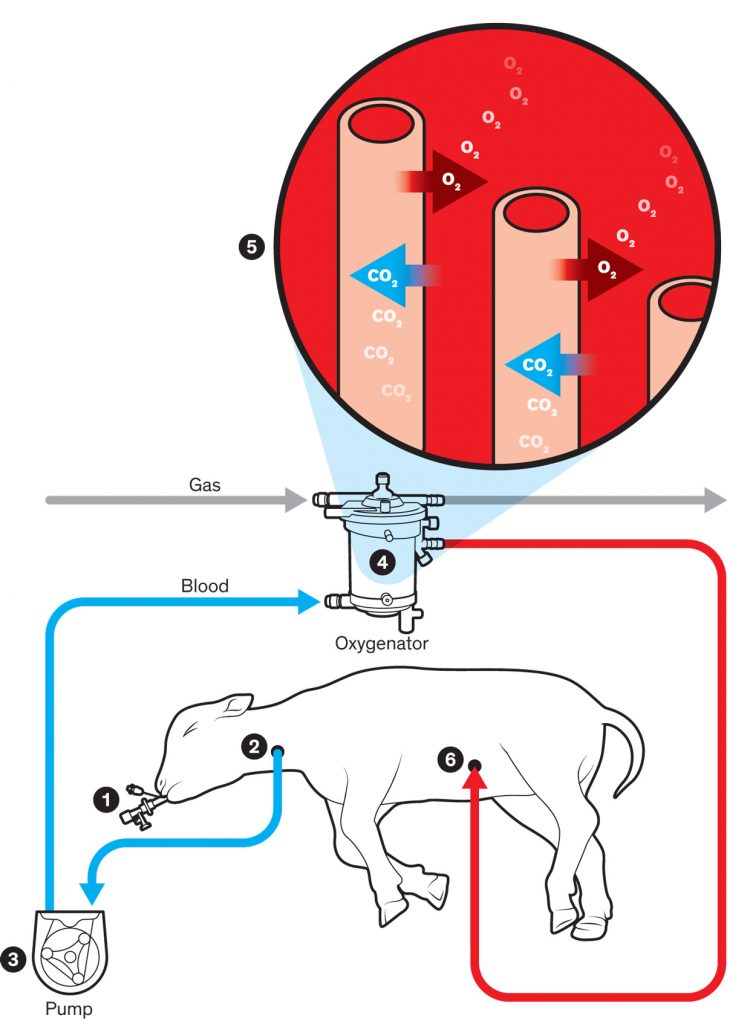
The system devised by George Mychaliska’s team performs the most important function of a placenta: providing oxygen and taking away carbon dioxide. In the team’s artificial-placenta system, the lamb’s lungs continue to be filled with fluid, as they would be in the womb, and a clamped endotracheal tube (1) ensures that the lamb won’t start breathing and damage its premature lungs. A tube is inserted into the jugular vein (2), and a pump (3) ensures a steady flow of blood out of the body and into the oxygenator (4). Within the oxygenator, the blood flows around hollow fibers (5) filled with oxygen, and red blood cells receive this oxygen through the fibers’ membranes and give carbon dioxide in return. The oxygenated blood then flows back into the lamb through a tube (6) in an umbilical-cord vein. A nitric oxide coating within the tubing and nitric oxide gas within the oxygenator prevent blood from clotting as it passes through.
But ECMO hadn’t been used on extremely premature babies younger than 28 weeks, and survival rates for those tiny creatures remained poor. When Mychaliska was recruited to the University of Michigan 15 years ago to build a fetal surgery program, he immediately became interested in Bartlett’s work. He knew that physicians hadn’t tried ECMO technology on extreme preemies because they feared that the systems were simply too big for tiny infants, who can weigh less than 500 grams. What’s more, ECMO usually requires doses of the blood thinner heparin to prevent clotting inside the circuitry—a no-go for extreme preemies because they’re already predisposed to bleeding complications. Still, Mychaliska was willing to try to see if the oxygenators could be adapted for use in extreme preemies.
The first step was to get the system to work with 118-day-old lamb fetuses, whose lungs are at a similar developmental stage as those of 24-week-old human fetuses. During the researchers’ first experiments in 2007, they connected the animals to an ECMO oxygenator in the same way the fetuses would be connected to a placenta. Blood from each lamb’s umbilical artery flowed out through a plastic tube called a cannula, moved through the oxygenator, and then flowed back into the lamb’s body through a cannula in the umbilical vein. Mychaliska hoped that the lambs’ hearts would pump blood through the artificial system. But one by one, the lambs’ blood flow slowed down. Two had heart failures. “We couldn’t maintain support much beyond 4 hours,” he remembers.
Mychaliska’s team discovered that the lambs’ removal from the womb had naturally triggered the umbilical artery to constrict, which caused the whole system to grind to a halt. The researchers got around this problem by using each animal’s jugular vein to channel blood into the oxygenator. Because blood in the veins isn’t forcefully propelled by the heartbeat (as it is in arteries), they had to add a roller pump to keep the blood flowing. This approach would take pressure off the developing heart should the resistance in the artificial circuit be higher than in the natural placenta, the team reasoned.
As the tiny lambs lay inside incubators, their lungs filled with fluid—as the lungs would be naturally at that stage of development—they were monitored around the clock by doctors and students, who readjusted the pumps and oxygenators to keep oxygen concentrations steady and at levels appropriate for a premature creature. The support staff gave the animals hourly infusions of protein, sugars, vitamins, and other nutrients. Other than the fact that the lambs were breathing through a device that was mounted on a pole beside them, they behaved like any other infants: suckling, moving, and sometimes kicking around. “Just like any baby in an ICU, you have to be sure they don’t do themselves damage,” Bartlett says.
By 2014, Mychaliska and his colleagues had the system working well enough to sustain the lambs for a week. They’ve since stretched that span to three weeks, according to recent unpublished data. By contrast, premature lambs that were put on a ventilator tended to succumb within 8 hours. To ensure the lambs had developed normally on the artificial placenta, the team autopsied euthanized animals, carefully dissecting their lungs, brains, and hearts. So far, the results look good.

Prepping for Preemies: The artificial-placenta system developed by George Mychaliska’s team has kept extremely premature lambs alive for weeks. He predicts that trials with human preemies will commence in five years.
Other research groups have demonstrated similar results. In 2017, physicians at the Children’s Hospital of Philadelphia (CHOP) Research Institute announced they had successfully sustained extremely premature lambs, the equivalent of 23- or 24-week-old human babies, for up to four weeks. Inside their “biobag” system, fetuses are submerged in an amniotic-like fluid, creating a more natural womblike environment. The achievement by the CHOP researchers, who declined to comment for this article, sparked intense media coverage, including far-fetched speculation that the technology would one day replace pregnancy entirely.
Meanwhile, at the University of Western Australia, in Perth, perinatology researcher Matthew Kemp and his colleagues have developed what they call an ex vivo uterine environment therapy. In collaboration with researchers at Tohoku University and the Japanese medical device company Nipro Corp., Kemp’s team has been able to sustain tiny lambs (which are slightly younger than Mychaliska’s lambs) for a week. Like the CHOP team, Kemp has worked out how to connect his system to the umbilical artery without causing it to constrict—though the doctors will have to act exceedingly fast. Both systems use the fetus’s own heart to pump blood, which is more natural, but they also come “with a range of challenges because the fetal heart is pretty easily damaged,” Kemp says.
While these results show promise, there are several major challenges. For one thing, the machines still can’t be used on human infants, who can’t be placed on blood thinners. Blood is an engineer’s nightmare for several reasons, but particularly because it begins to clot once it encounters any unnatural surface. Patients on ECMO are administered heparin, which is fine for typical patients, and apparently also the lambs in Mychaliska’s experiments. But it’s an unacceptable risk for infants younger than 28 weeks, as the barriers between blood vessels and developing neural tissue aren’t yet mature. Preventing the blood from clotting could easily cause fatal bleeding into the brain. “That’s been one of the elephants in the room for this work,” Mychaliska says.
All the groups have grappled with this problem. For Mychaliska’s team, the breakthrough came from University of Michigan chemist Mark Meyerhoff. Blood doesn’t typically clot as it circulates through the human body partly because the lining of the blood vessels emits a continuous stream of nitric oxide gas, which prevents the blood from coagulating. Meyerhoff, Bartlett, and colleagues developed a synthetic polymer that emits nitric oxide, and worked it into a coating for plastic tubing. Combined with a device that releases nitric oxide into the oxygenator itself—since any coating there would disrupt the biological gas exchange—the result is an entire oxygenation system that doesn’t require heparin. For all practical purposes, the blood will behave as if it were still in the body. Mychaliska says that recent experiments to test the coated system with premature lambs were promising.
Perhaps the biggest challenge that the teams are wrestling with is just how tiny premature human babies are. Premature lambs can weigh a few kilograms more than human infants do at a similar stage of lung development, making them an imperfect analogue. The blood vessels of human preemies can be only 2 or 3 millimeters wide, meaning that the cannulas used to connect the devices must be extraordinarily slim. But as the cannulas get smaller, the pressure drop inside the cannulas and tubing increases exponentially. That means the device’s pump—whether it’s an artificial one or the fetus’s heart—will have to work much harder to push fluid through, a situation that researchers want to avoid. “There still is a fair amount of bioengineering and experimentation that needs to be done to demonstrate that you can maintain adequate flows with smaller catheters,” Mychaliska says.
Tech to Save Preemies
Extremely premature infants—those born before 28 weeks—often don’t survive, and those that do frequently have problems with their underdeveloped lungs. Several research groups are developing technologies that can support preemies until they can breathe on their own, focusing particularly on infants at the very limits of viability—around 23 or 24 weeks. The systems haven’t yet been tested on humans, but animal tests have been promising.
| WHAT’S THE TECHNOLOGY? | ARTIFICIAL PLACENTA | BIOBAG | EX VIVO UTERINE ENVIRONMENT THERAPY | MICROFLUIDIC ARTIFICIAL PLACENTA |
|---|---|---|---|---|
| WHO’S DEVELOPING IT? | University of Michigan C.S. Mott Children’s Hospital | Children’s Hospital of Philadelphia (CHOP) Research Institute | University of Western Australia, Tohoku University, and Nipro Corp. | McMaster University and Nuremberg Hospital |
| HOW DOES IT WORK? | The artificial placenta relies on an oxygenator used in extracorporeal membrane oxygenation (ECMO) therapy to provide oxygen to the premature infant, who lies inside an incubator. | While connected to an ECMO oxygenator, the infant is submerged in a womblike fluid inside a bag. | While connected to an oxygenator, the infant is submerged in a womblike fluid inside a bag. | This system uses a novel kind of oxygenator, which, by mimicking blood-vessel structures in the lung, allows blood to take up oxygen from the air directly. The infant can breathe while connected to the device. |
| HOW DOES THE BLOOD FLOW? | This system uses a mechanical pump to shunt blood through the tubing. | The infant’s own heart drives blood through the system. | The infant’s own heart drives blood through the system. | The infant’s own heart drives blood through the system. |
| HOW DOES IT CONNECT TO THE INFANT? | The system takes blood from the jugular vein in the neck and returns it via the umbilical vein. | The system uses the umbilical arteries and the umbilical vein. | The system uses the umbilical arteries and the umbilical vein. | The system uses the umbilical arteries and the umbilical vein. |
| HOW DOES IT PREVENT BLOOD CLOTTING? | It uses a special coating that prevents blood clotting inside the tubing, thereby avoiding the use of blood-thinning drugs, which preemies can’t tolerate. | The research team hasn’t yet published any data on this topic. | The group is developing coatings to avoid the use of blood thinners. | Researchers are testing several types of coatings to avoid the use of blood thinners. |
| HOW FAR ALONG IS THE RESEARCH? | The artificial placenta can currently sustain premature lambs for several weeks. The researchers expect to test the technology in human infants within five years. | 2017 research demonstrated that the biobag could sustain premature lambs for up to one month. The team says it hopes to launch human trials in the next few years. | The team has demonstrated that its approach can keep premature lambs alive for one week. | This system is still in the early stages of testing. In a recent proof-of-concept study, it oxygenated a sick piglet for several hours. |
On top of that, the volume of most commercial oxygenators is simply too large, notes Jutta Arens, a biomechanical engineer at the University of Twente, in the Netherlands, who is part of a European network developing an artificial placenta. Today’s devices might work for heavier infants with a total of a few hundred milliliters of blood, but not for, say, a 600-gram infant with only 60 milliliters of blood. When adults are hooked up to ECMO, saline is used to “prime” the system by chasing out air, and upon flowing into the patient, it harmlessly dilutes their blood. With preemies, however, there’s a limit to how much saline their blood can tolerate before losing the ability to transport oxygen. Donated adult blood is also not ideal, as it has entirely different gas-exchange properties.
Around the world, the teams working on artificial placentas are taking different approaches to that problem. For now, Mychaliska’s team is seeing how far they can get with commercial oxygenators, while Arens’s group in the Netherlands and Kemp’s group in Australia are designing smaller oxygenators and tubing tailored to these tiny patients.
At Canada’s McMaster University, in Hamilton, biomedical engineer Ravi Selvaganapathy is collaborating with a team at Nuremberg Hospital, in Germany, on an entirely new kind of oxygenator, using the lung as a blueprint. Lungs are such efficient gas exchangers because of the architecture of their blood vessels: Large incoming branches split into innumerable tiny twigs, each a few micrometers thick. Those twigs are just large enough for individual red blood cells to squeeze through one at a time, allowing them to take up as much oxygen as possible.
Similarly, the McMaster-Nuremberg system breaks out into a gridlike network of tiny tubes made from a gas-permeable silicone, through which red blood cells absorb oxygen from the atmosphere itself. “It’s just exactly like the lung—it is air-breathing,” Selvaganapathy says. His team reported last year that this microfluidic approach successfully kept a small sick piglet alive for several hours. Another advantage, according to Niels Rochow, a German neonatologist on the project, is that oxygenator units can be added, if necessary, to increase gas-exchange capacity as the preemie grows.
Designing systems for the most delicate humans means balancing many considerations. Pumpless designs that rely on the preemie’s heart might not work for babies with heart problems. But Rochow notes that artificial pumps can damage oxygen-carrying red blood cells. “You can’t tell today which is the right approach,” Arens says. “It might turn out that we are all on the right path, but for different patients.”
Mychaliska expects his device to be tested in human infants within the next five years, while the CHOP team says just two years for theirs. But others are skeptical. “I think they need to do a lot more testing before thinking about this as an artificial womb for clinical use,” says Anita Moon-Grady, who directs the fetal cardiovascular program at the University of California, San Francisco.

Living Proof: “Large Marge” was bigger than the lambs typically connected to the artificial placenta system. At the conclusion of the experiment, she went to live on a farm, where she went on to produce two healthy offspring.
Anna Penn, a neonatologist and developmental neuroscientist at Columbia University, says it’s unclear how transferable the results from sheep will be to humans. Sheep brains develop at a different rate than human brains, and their umbilical cords are much shorter and straighter than human ones; both factors could affect how well the devices work in people. Penn thinks artificial placentas will eventually enter the clinic, but whether they become a widespread part of neonatal intensive care will depend on the long-term benefits and risks. Infants get a lot more from the placenta than nutrition and oxygen, including hormones and other substances that scientists are only just beginning to understand, she notes. “I think it would be wonderful if something can be developed that works extremely well, [but I think it’s] unlikely that they will ever be as effective as a healthy functioning placenta.”
For bioethicists, too, artificial placentas pose tricky questions. If the devices work as intended—helping 24-week old babies become healthy 28-week-olds—it’s hard to see why anybody would have a problem with them, notes Dena Davis, a bioethicist at Lehigh University, in Pennsylvania. But problems could arise if the technology is used on even younger fetuses, she says. In the United States, the legal grounding for abortion rests on a notion of viability—that is, an ability to exist outside a woman’s body. Most doctors say that a fetus is theoretically viable at about 24 weeks. If artificial placentas are used in younger fetuses, “then that gives antichoice people more ammunition for saying, ‘Well, you know, even in the second trimester, a baby can be viable,’ ” Davis says.
Other bioethicists, as well as some scientists, raise a much bigger issue: With in-vitro fertilization now commonplace and artificial placentas in development, might technology eventually close the gap and lead to true artificial wombs, in which babies are grown without mothers? Media coverage of artificial-placenta research has often evoked Brave New World, Aldous Huxley’s 1932 vision of a dystopian future where people are mass-produced in “decanting bottles” by an authoritarian state, or the 1999 movie The Matrix, where people are manufactured in fluid-filled pods.
Technologically speaking, those sci-fi fantasies remain exactly that—fantasies. Arens, of the University of Twente, says that the inability to cannulate tiny blood vessels alone makes it impossible to design an artificial placenta for any infant much younger than 22 or 23 weeks. And despite thousands of scientific papers exploring the earliest stages of human development, much remains mysterious about how the womb creates the perfect conditions for a fertilized egg to grow into a fetus. Re-creating that artificially won’t be possible anytime soon, says Graham Burton, a developmental biologist at the University of Cambridge.
Mychaliska, for his part, has no time for fantasy. He prefers the reality that’s currently living on a farm in Michigan. Years ago, the team decided to let one sturdy-looking lamb on the artificial placenta wean onto room air; then they bottle-fed her for about a week and donated her to a farm. Removed from the womb at 130 days, Large Marge had been much older than the lambs usually connected to the system, and only spent three days under its support. But the fact that she went on to live five healthy years—and even left behind two youngsters—demonstrates what Mychaliska hopes to achieve someday in people. For him, it’s not just about preventing death but enabling a healthy life—and the chance to produce new life. “That’s the dream,” he says. “Truthfully, I think we’re getting very close.”
Source: www.spectrum.ieee.org






















































